Abstract
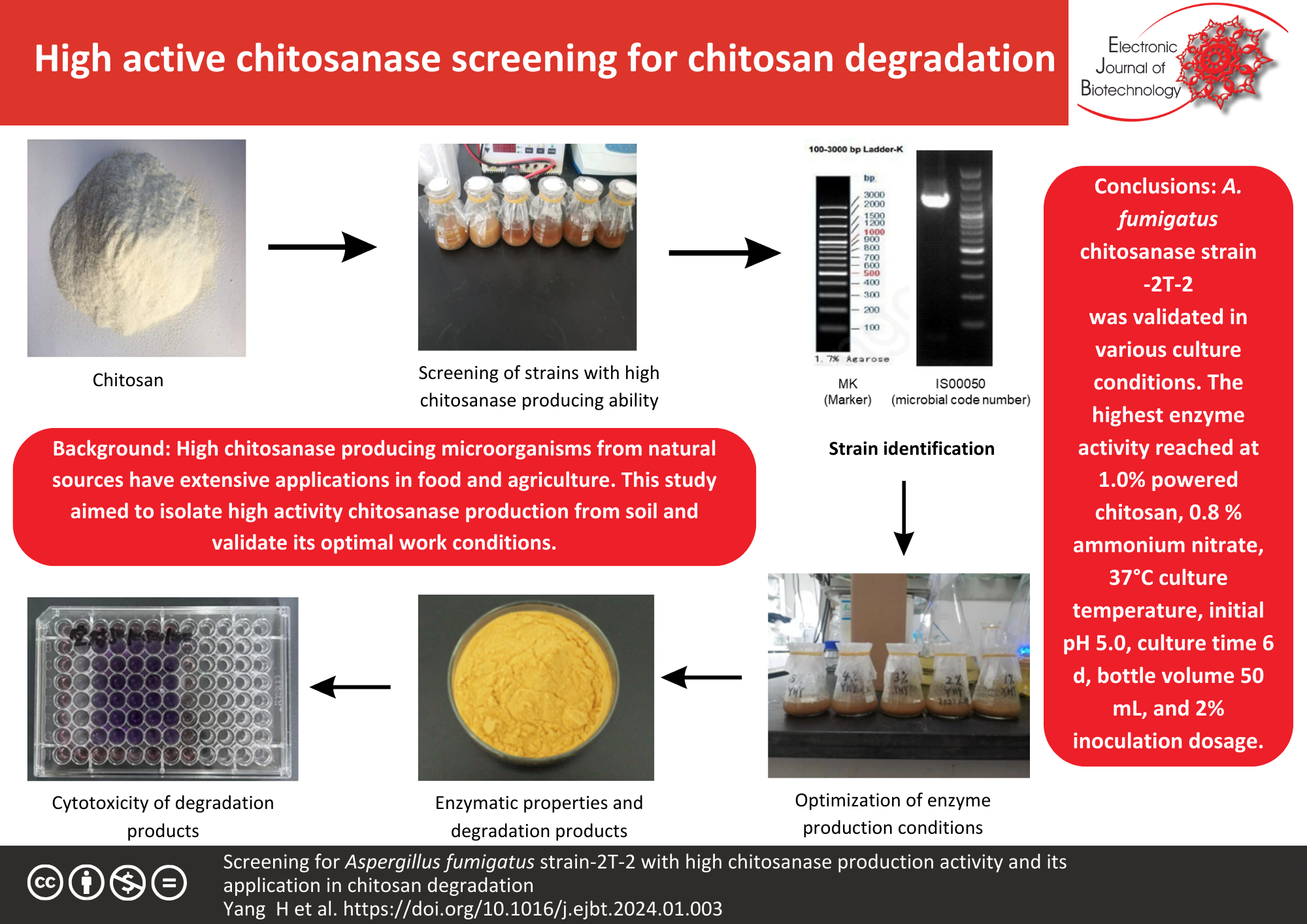
Background: High chitosanase-producing microorganisms from natural sources have extensive applications in food and agriculture. This study aimed to optimal conditions for high-activity chitosanase production. A named CGMCC21422 chitosanase-producing strain -2T-2 was isolated from soil and identified named as Aspergillus fumigatus chitosanase (A. fumigatus chitosanase). This enzymatic activity was validated in various culture conditions. It is stored in the China General Microbiological Culture Collection Center. The efficacy of A. fumigatus chitosanase in the degradation of chitosan was validated.
Results: In this study, we determined that the optimal fermentation conditions of stain-2T-2 were 1.0% powered chitosan, 0.8% ammonium nitrate, 37°C culture temperature, initial pH 5.0, culture time 6 d, bottle volume 50 mL, and 2% inoculation dosage. Under these culture conditions, the highest enzyme activity of fermentation broth in the shaker flask reached 827.53 U/mL. The optimal reactive conditions of A. fumigatus-produced chitosanase are 55–60°C and pH 4.5. When the reactive temperature was over 60°C, the A. fumigatus chitosanase was easily inactivated. The chitosanase catalyzed substrate chitosan to produced ≈20% chito-oligosaccharide and ≥80% glucosamine salt samples in a variety of acidic solutions. These reactive products are not cytotoxic or mild to MH7A cells.
Conclusions: A. fumigatus chitosanase strain -2T-2 is a strain with high chitosanase and can catalyze chitosan into chito-oligosaccharide in acidic solutions.
References
Guan G, Azad MAK, Lin Y, et al. Biological effects and applications of chitosan and chito-oligosaccharides. Front Physiol. 2019;10:516. https://doi.org/10.3389/fphys.2019.00516 PMid: 31133871
Swiatkiewicz S, Swiatkiewicz M, Arczewska-Wlosek A, et al. Chitosan and its oligosaccharide derivatives (chito-oligosaccharides) as feed supplements in poultry and swine nutrition. J Anim Physiol Anim Nutr 2015;99(1):1-12. https://doi.org/10.1111/jpn.12222 PMid: 25041091
Chen D, Chen C, Zheng X, et al. Chitosan oligosaccharide production potential of Mitsuaria sp. C4 and its whole-genome sequencing. Front Microbiol. 2021;12:695571. https://doi.org/10.3389/fmicb.2021.695571 PMid: 34421850
Sacco P, Cok M, Scognamiglio F, et al. Glycosylated-chitosan derivatives: A systematic review. Molecules. 2020;25(7):1534. https://doi.org/10.3390/molecules25071534 PMid: 32230971
Kaczmarek MB, Struszczyk-Swita K, Li X, et al. Enzymatic modifications of chitin, chitosan, and chitooligosaccharides. Front Bioeng Biotechnol. 2019;7:243. https://doi.org/10.3389/fbioe.2019.00243 PMid: 31612131
Kou SG, Peters LM, Mucalo MR. Chitosan: A review of sources and preparation methods. Int J Biol Macromol. 2021;169:85-94. https://doi.org/10.1016/j.ijbiomac.2020.12.005 PMid: 33279563
Younes I, Rinaudo M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar Drugs. 2015;13(3):1133-74. https://doi.org/10.3390/md13031133 PMid: 25738328
Zhang J, Cai K, Mishra R, et al. In ovo supplementation of chitooligosaccharide and chlorella polysaccharide affects cecal microbial community, metabolic pathways, and fermentation metabolites in broiler chickens. Poult Sci. 2020;99(10):4776-85. https://doi.org/10.1016/j.psj.2020.06.061 PMid: 32988512
Oyeleye A, Normi YM. Chitinase: Diversity, limitations, and trends in engineering for suitable applications. Biosci Rep. 2018;38(4). https://doi.org/10.1042/BSR20180323 PMid: 30042170
Jung WJ, Park RD. Bioproduction of chitooligosaccharides: Present and perspectives. Mar Drugs. 2014;12(11):5328-56. https://doi.org/10.3390/md12115328 PMid: 25353253
Zhou J, Wen B, Xie H, et al. Advances in the preparation and assessment of the biological activities of chitosan oligosaccharides with different structural characteristics. Food Funct. 2021;12(3):926-51. https://doi.org/10.1039/D0FO02768E PMid: 33434251
Yorinaga Y, Kumasaka T, Yamamoto M, et al. Crystal structure of a family 80 chitosanase from Mitsuaria chitosanitabida. FEBS Lett. 2017;591(3):540-7. https://doi.org/10.1002/1873-3468.12557 PMid: 28084023
Li Y, Gou Y, Liu Z, et al. Structure-based rational design of chitosanase CsnMY002 for high yields of chitobiose. Colloids Surf B Biointerfaces. 2021;202:111692. https://doi.org/10.1016/j.colsurfb.2021.111692 PMid: 33744813
Jiang X, Chen D, Chen L, et al. Purification, characterization, and action mode of a chitosanase from Streptomyces roseolus induced by chitin. Carbohydr Res. 2012;355:40-4. https://doi.org/10.1016/j.carres.2012.05.002 PMid: 22647542
Su C, Zhou W, Fan Y, et al. Mutation breeding of chitosanase-producing strain Bacillus sp. S65 by low-energy ion implantation. J Ind Microbiol Biotechnol. 2006;33(12):1037-42. https://doi.org/10.1007/s10295-006-0155-7 PMid: 16897082
Lodhi G, Kim YS, Hwang JW, et al. Chitooligosaccharide and its derivatives: Preparation and biological applications. Biomed Res Int. 2014;2014:654913. https://doi.org/10.1155/2014/654913 PMid: 24724091
Jung WJ, Kuk JH, Kim KY, et al. Purification and characterization of exo-?-?-glucosaminidase from Aspergillus fumigatus S-26. Protein Expr Purif. 2006;45(1):125-31. https://doi.org/10.1016/j.pep.2005.06.016 PMid: 16289917
Cheng CY, Chang CH, Wu YJ, et al. Exploration of glycosyl hydrolase family 75, a chitosanase from Aspergillus fumigatus. J Biol Chem. 2006;281(6):3137-44. https://doi.org/10.1074/jbc.M512506200 PMid: 16330537
Nguyen AD, Huang CC, Liang TW, et al. Production and purification of a fungal chitosanase and chitooligomers from Penicillium janthinellum D4 and discovery of the enzyme activators. Carbohydr Polym. 2014;108:331-7. https://doi.org/10.1016/j.carbpol.2014.02.053 PMid: 24751281
Choi YJ, Kim EJ, Piao Z, et al. Purification and characterization of chitosanase from Bacillus sp. strain KCTC 0377BP and its application for the production of chitosan oligosaccharides. Appl Environ Microbiol. 2004;70(8):4522-31. https://doi.org/10.1128/AEM.70.8.4522-4531.2004 PMid: 15294781
Fukamizo T, Shinya S. Chitin/chitosan-active enzymes involved in plant-microbe interactions. Adv Exp Med Biol. 2019;1142:253-72. https://doi.org/10.1007/978-981-13-7318-3_12 PMid: 31102250
Hao W, Li K, Ma Y, et al. Preparation and antioxidant activity of chitosan dimers with different sequences. Mar Drugs. 2021;19(7). https://doi.org/10.3390/md19070366 PMid: 34201994
Fukamizo T, Brzezinski R. Chitosanase from Streptomyces sp. strain N174: A comparative review of its structure and function. Biochem Cell Biol. 1997;75(6):687-96. https://doi.org/10.1139/o97-079 PMid: 9599657
Adachi W, Sakihama Y, Shimizu S, et al. Crystal structure of family GH-8 chitosanase with subclass II specificity from Bacillus sp. K17. J Mol Biol. 2004;343(3):785-95. https://doi.org/10.1016/j.jmb.2004.08.028 PMid: 15465062
Hirano K, Arayaveerasid S, Seki K, et al. Characterization of a chitosanase from Aspergillus fumigatus ATCC13073. Biosci Biotechnol Biochem. 2012;76(8):1523-8. https://doi.org/10.1271/bbb.120248 PMid: 22878198
Omumasaba CA, Yoshida N, Sekiguchi Y, et al. Purification and some properties of a novel chitosanase from Bacillus subtilis KH1. J Gen Appl Microbiol. 2000;46(1):19-27. https://doi.org/10.2323/jgam.46.19 PMid: 12483600
Ji K, Li H, Gong J, et al. Preparation of chitosan oligosaccharides by enzymatic hydrolysis and evaluation of antitumor activities. Journal of Food Science and Biotechnology 40(6): 93-99. 2021;40(6):93-9.
Das SN, Wagenknecht M, Nareddy PK, et al. Amino groups of chitosan are crucial for binding to a family 32 carbohydrate binding module of a chitosanase from Paenibacillus elgii. J Biol Chem. 2016;291(36):18977-90. https://doi.org/10.1074/jbc.M116.721332 PMid: 27405759
Wang X, Li X, Guo M, et al. Research progress of clinical effects of oral and external application of TCM combined with aminoglucose in the treatment of knee osteoarthritis. Western Journal of Traditional Chinese Medicine 2022;35(1):149-52.

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright (c) 2024 Electronic Journal of Biotechnology