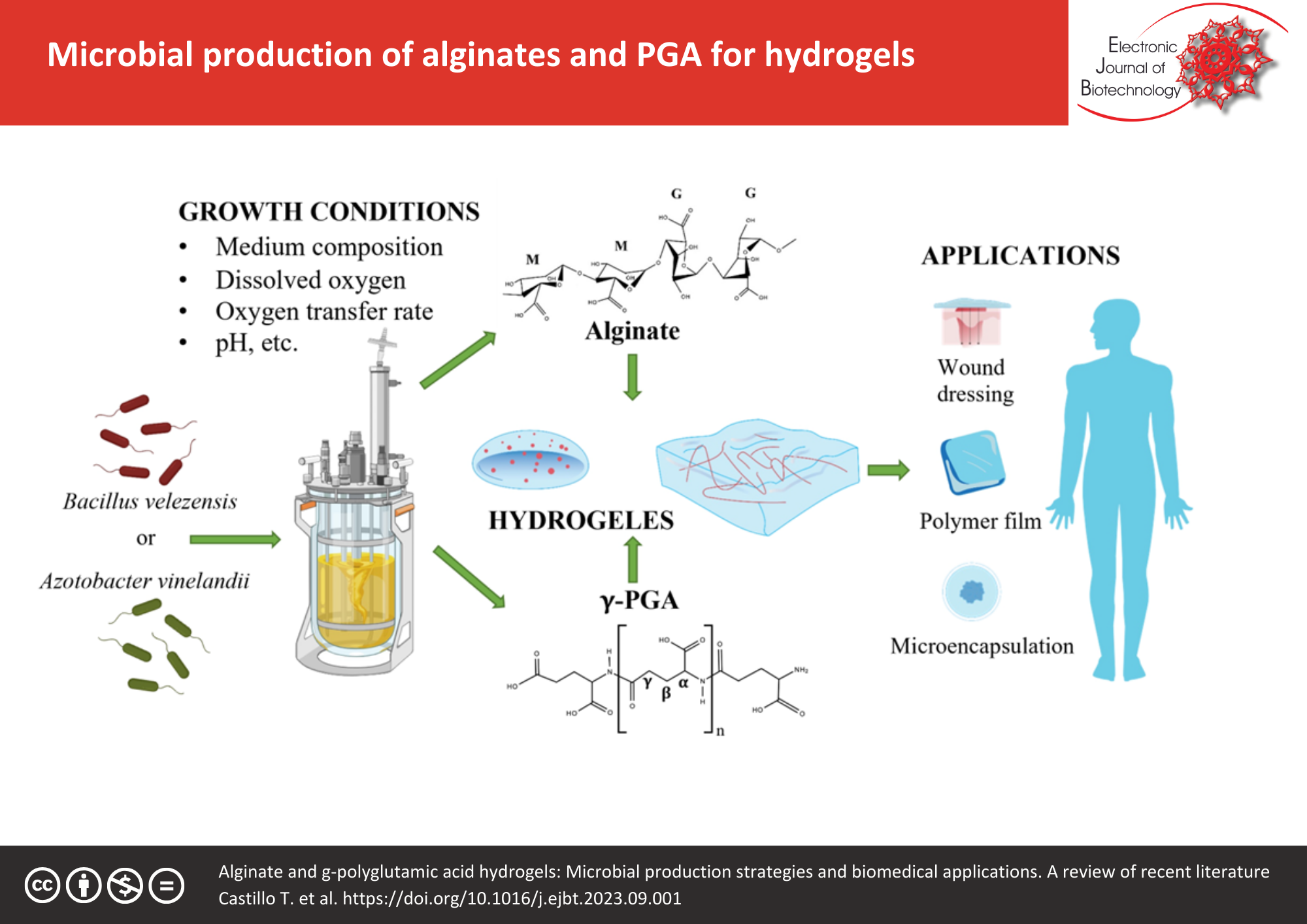
Abstract
Hydrogels are three-dimensional networks of hydrophilic polymers. In general, these structures can be soft, elastic, porous and can absorb high quantities of water. Due to these characteristics, there is a growing interest in the use of hydrogels in diverse areas, from bioremediation to applications in the biomedical field. Although hydrogels can be elaborated with natural and synthetic polymers, natural polymers are attracting attention for their use in the biomedical and pharmaceutical fields. Alginate and γ-polyglutamic acid (γ-PGA) are microbial polymers, which show a great potential for hydrogel elaboration because of their biocompatibility that positioned them in emerging technologies, such as tissue engineering, microencapsulation, and soft robotics; these applications require specific characteristics of hydrogels in terms of their mechanical resistance, swelling capability, flexibility, softness, and stiffness. Thus, there is an emerging interest in the microbial production of alginates and γ-PGA, where it is possible to change their physicochemical and thermomechanical characteristics by the manipulation of the culture growth conditions of the microbial producers that can be oriented to specific applications. In this review, the chemical composition of biopolymers, hydrogel structure, the applications of hydrogels of alginates and γ-PGA, as well as their advantages and limitations are described; besides, the bacterial production of these polymers and the growth conditions that modify their chemical composition, are discussed.
References
Hu Y, Hu S, Zhang S, et al. A double-layer hydrogel based on alginate-carboxymethyl cellulose and synthetic polymer as sustained drug delivery system. Sci Rep 2021;11:9142. https://doi.org/10.1038/s41598-021-88503-1.
Slaughter BV, Khurshid SS, Fisher OZ, et al. Hydrogels in regenerative medicine. Adv Mater 2009;21:3307–29. https://doi.org/10.1002/adma.200802106.
Wang L, Li Y, Du X, et al, Performance enhancement of white rot fungi extracellular enzymes via new hydrogel microenvironments for remediation of benzo[a]pyrene contaminated soil. J Hazard Mater 2023;454.131505, https://doi.org/10.1016/j.jhazmat.2023.131505 .
Chen Y, Zhang W, Ding X, et al. Programmable scaffolds with aligned porous structures for cell cultured meat. Food Chem 2024;430:137098. https://doi.org/10.1016/j.foodchem.2023.137098.
Enawgaw H, Tesfaye T, Yilma KT, et al. Synthesis of a cellulose-Co-AMPS hydrogel for personal hygiene applications using cellulose extracted from corncobs. Gels 2021;7. https://doi.org/10.3390/gels7040236.
Barbosa I T F, Oliveira B, Rocha G, et al. Characterization of hydrogels containing mandelic acid nanoemulsions and different essential oils. Mater Res. 2023; 26: e20220619. doi.org/10.1590/1980-5373-MR-2022-0619.
Chai N, Zhang J, Zhang Q, et al. Construction of 3D printed constructs based on microfluidic microgel for bone regeneration. Compos B Eng 2021;223:109100. https://doi.org/10.1016/j.compositesb.2021.109100.
Elkhoury K, Morsink M, Sanchez-Gonzalez L, et al. Biofabrication of natural hydrogels for cardiac, neural, and bone tissue engineering applications. Bioact Mater 2021;6:3904–23. https://doi.org/10.1016/j.bioactmat.2021.03.040.
Wang L, Zhang HJ, Liu X, et al. A physically cross-linked sodium alginate–gelatin hydrogel with high mechanical strength. ACS Appl Polym Mater 2021;3:3197–205. https://doi.org/10.1021/acsapm.1c00404.
Zhang X, Li Y, Ma Z, et al. Modulating degradation of sodium alginate/bioglass hydrogel for improving tissue infiltration and promoting wound healing. Bioact Mater 2021;6:3692–704. https://doi.org/10.1016/J.BIOACTMAT.2021.03.038.
Du Y, Chen X, Li L, et al. Benzeneboronic?alginate/quaternized chitosan?catechol powder with rapid self-gelation, wet adhesion, biodegradation and antibacterial activity for non-compressible hemorrhage control. Carbohyd Polym 2023;318:121049. https://doi.org/10.1016/j.carbpol.2023.121049.
Luo M, Huang M, Yang N, et al. Impairment of rigidity sensing caused by mutant TP53gain of function in osteosarcoma. Bone Res 2023;11,28. https://doi-org.pbidi.unam.mx:2443/10.1038/s41413-023-00265-w
Xie X, Xu Z, Yu X, et al. Liquid-in-liquid printing of 3D and mechanically tunable conductive hydrogels. Nat Commun 2023;14,4289. https://doi.org/10.1038/s41467-023-40004-7
Sun Z, Zhu D, Zhao H. et al. Recent advance in bioactive hydrogels for repairing spinal cord injury: material design, biofunctional regulation, and applications. J Nanobiotechnol 2023;21,238. https://doi.org/10.1186/s12951-023-01996-y
Xu T, Wang J, Zhao S. et al. Accelerating the prediction and discovery of peptide hydrogels with human-in-the-loop. Nat Commun 2023;14,3880 https://doi-org.pbidi.unam.mx:2443/10.1038/s41467-023-39648-2
Ma X, Liu S, Tang H, et al. In situ photocrosslinked hyaluronic acid and poly (?-glutamic acid) hydrogels as injectable drug carriers for load-bearing tissue application. J Biomater Sci Polym Ed 2018;29:2252. https://doi.org/10.1080/09205063.2018.1535820.
Gryshkov O, Mutsenko V, Tarusin D, et al. Coaxial alginate hydrogels: from self-assembled 3D cellular constructs to long-term storage. Int J Mol Sci 2021;22:3096. https://doi.org/10.3390/ijms22063096.
Sahoo DR, Biswal T. Alginate and its application to tissue engineering. SN Appl Sci 2021;3:30. https://doi.org/10.1007/s42452-020-04096-w.
Peña C, Galindo E, Büchs J. The viscosifying power, degree of acetylation and molecular mass of the alginate produced by Azotobacter vinelandii in shake flasks are determined by the oxygen transfer rate. Process Biochem 2011; 46:290–7. https://doi.org/10.1016/j.procbio.2010.08.025.
Cao M, Feng J, Sirisansaneeyakul S, et al. Genetic and metabolic engineering for microbial production of poly-?-glutamic acid. Biotechnol Adv 2018;36:1424–33. https://doi.org/10.1016/j.biotechadv.2018;05.006.
Park S-J, Uyama H, Kwak M-S, et al. Comparison of the stability of poly-g-glutamate hydrogels prepared by UV and g-Ray Irradiation. J Microbiol Biotechnol 2019;29:1078–82. https://doi.org/10.4014/jmb.1812.12018.
Yu Z, Li Q, He X, et al. A multifunctional hydrogel based on nature polysaccharide fabricated by Schiff base reaction. Eur Polym J 2023;197:112330, https://doi.org/10.1016/j.eurpolymj.2023.112330.
Hong N, Yang G-H, Lee JH, et al. 3D bioprinting and its in vivo applications. J Biomed Mater Res Part B 2018; 106B: 444–459.
Gulyuz U. Dual cross-linked polymethacrylic acid hydrogels with tunable mechanical properties and shape memory behavior. Macromol Mater Eng 2021;306: 2100201. https://doi.org/10.1002/mame.202100201
Casey E, Kandow PC, Georges PA, et al. Polyacrylamide hydrogels for cell mechanics: steps toward optimization and alternative uses. Methods Cell Biol 2007;83:29-46. https://doi.org/10.1016/S0091-679X(07)83002-0.
Konwar A, Gogoi N, Majumdar G, Chowdhury D, Green chitosan-carbon dots nanocomposite hydrogel film with superior properties. Carbohydr Polym 2015;115, 238–245. doi: 10.1016/j.carbpol.2014.08.021.
Wang J-H, Tsai C-W, Tsai N-Y, et al. An injectable, dual crosslinkable hybrid pectin methacrylate (PECMA)/gelatin methacryloyl (GelMA) hydrogel for skin hemostasis applications. Int J Biol Macromol 2021;185: 441-450, https://doi.org/10.1016/j.ijbiomac.2021.06.162.
Aminabhavi TM, Dharupaneedi SP. Production of chitosan-based hydrogels for biomedical applications. In: Jennings JA, Bumgardner JD, editors. Chitosan based biomaterials. Volume 1, Fundamentals. United Kingdom: Elsevier; 2017, p. 295 – 313.
Cheng Y, Chan KH, Wang X-Q, et al. Direct-ink-write 3D printing of hydrogels into biomimetic soft robots. ACS Nano 2019;13:13176–84. https://doi.org/10.1021/acsnano.9b06144.
Sarangi MK, Rao MEB, Parcha V, et al. Marine polysaccharides for drug delivery in tissue engineering. In: Hasnain MS, Nayek AK, editors. Natural polysaccharides in drug delivery and biomedical applications. United Kingdom: Academic Press; 2019, p. 513 – 530.
Sánchez M, Vásquez-Quitral P, Butto N, et al. Effect of alginate from chilean Lessonia nigrescens and MWCNTs on CaCO3 crystallization by classical and non-classical methods. Crystals 2018;8:69. https://doi.org/10.3390/cryst8020069.
McKee JWA, Kavalieris L, Brasch DJ, et al. Alginate content and composition of Macrocystis pyrifera from New Zealand. J Appl Phycol 1992;4:357–69. https://doi.org/10.1007/BF02185794.
Urtuvia V, Maturana N, Acevedo F, et al. Bacterial alginate production: an overview of its biosynthesis and potential industrial production. World J Microbiol Biotechnol 2017;33:198. https://doi.org/10.1007/s11274-017-2363-x.
Klöck G, Frank H, Houben R, et al. Production of purified alginates suitable for use in immunoisolated transplantation. Appl Microbiol Biotechnol 1994;40:638–43. https://doi.org/10.1007/BF00173321.
Klöck G, Pfeffermann A, Ryser C, et al. Bio-compatibility of mannuronic acid-rich alginates. Biomater 1997;18:707–13. https://doi.org/10.1016/S0142-9612(96)00204-9.
King A, Strand B, Rokstad A-M, et al. Improvement of the biocompatibility of alginate/poly-L-lysine/alginate microcapsules by the use of epimerized alginate as a coating. J Biomed Mater Res A 2003;64A:533–9. https://doi.org/10.1002/jbm.a.10276.
Wang P, Pu Y, Ren Y, et al. Dynamic regulable sodium alginate/poly(?-glutamic acid) hybrid hydrogels promoted chondrogenic differentiation of stem cells. Carbohydr Polym 2022;275:118692. https://doi.org/10.1016/j.carbpol.2021.118692.
Lee H, Yi GS, Nam Y. Connectivity and network burst properties of in-vitro neuronal networks induced by a clustered structure with alginate hydrogel patterning. Biomed Eng Lett. 2023. https://doi.org/10.1007/s13534-023-00289-5
Singh B, Kumar A, Rohit. Gamma radiation formation of sterculia gum-alginate-carbopol hydrogel dressing by grafting method for use in brain drug delivery. Chem Phys Lett 2021;779:138875. https://doi.org/10.1016/j.cplett.2021.138875.
Omtvedt LA, Kristiansen KA, Strand WI, et al. Alginate hydrogels functionalized with ??cyclodextrin as a local paclitaxel delivery system. J Biomed Mater Res A 2021;109:2625–39. https://doi.org/10.1002/jbm.a.37255.
Rehman N, Dilshad MR, Islam A, et al. Novel graphene oxide loaded sodium alginate hydrogels cross-linked with tetraethyl ortho-silicate for cephradine release analysis. J Drug Deliv Sci Technol 2021;66:102784. https://doi.org/10.1016/j.jddst.2021.102784.
Jung SW, Oh SH, Lee IS, et al. In situ gelling hydrogel with anti-bacterial activity and bone healing property for treatment of osteomyelitis. Tissue Eng Regen Med 2019;16:479–90. https://doi.org/10.1007/s13770-019-00206-x.
Hoefer D, Schnepf JK, Hammer TR, et al. Biotechnologically produced microbial alginate dressings show enhanced gel forming capacity compared to commercial alginate dressings of marine origin. J Mater Sci Mater Med 2015;26:162. https://doi.org/10.1007/s10856-015-5492-5.
Balogun-Agbaje OA, Odeniyi OA, Odeniyi MA. Drug delivery applications of poly-?-glutamic acid. Futur J Pharm Sci 2021;7:125. https://doi.org/10.1186/s43094-021-00280-w.
Nair P, Navale GR, Dharne MS. Poly-gamma-glutamic acid biopolymer: a sleeping giant with diverse applications and unique opportunities for commercialization. Biomass Conv Bioref. 2023;13,4555–73. https://doi.org/10.1007/s13399-021-01467-0.
Buescher JM, Margaritis A. Microbial biosynthesis of polyglutamic acid biopolymer and applications in the biopharmaceutical, biomedical and food industries. Crit Rev Biotechnol 2007;27:1–19. https://doi.org/10.1080/07388550601166458.
Chiesa E, Genta I, Dorati R, et al. Poly(gamma-glutamic acid) based thermosetting hydrogels for injection: rheology and functional parameters evaluation. React Func Polym. 2019;140:93–102. https://doi.org/10.1016/j.reactfunctpolym.2019.03.021.
Sirisansaneeyakul S, Cao M, Kongklom N, et al. Microbial production of poly-?-glutamic acid. World J Microbiol Biotechnol 2017;33:173. https://doi.org/10.1007/s11274-017-2338-y.
Yin M, Wang X, Yu Z, et al. ?-PGA hydrogel loaded with cell-free fat extract promotes the healing of diabetic wounds. J Mater Chem B 2020;8:8395–404. https://doi.org/10.1039/D0TB01190H.
Bajestani MI, Kader S, Monavarian M, et al. Material properties and cell compatibility of poly(?-glutamic acid)-keratin hydrogels. Int J Biol Macromol 2020;142:790–802. https://doi.org/10.1016/j.ijbiomac.2019.10.020.
Kuo Y-C, Ku H-F, Rajesh R. Chitosan/?-poly(glutamic acid) scaffolds with surface-modified albumin, elastin and poly- l -lysine for cartilage tissue engineering. Mater Sci Eng C 2017;78:265–77. https://doi.org/10.1016/j.msec.2017.04.067.
Yang N, Wang Y, Zhang Q, et al. ?-Polyglutamic acid mediated crosslinking PNIPAAm-based thermo/pH-responsive hydrogels for controlled drug release. Polym Degrad Stab 2017;144:53–61. https://doi.org/10.1016/j.polymdegradstab.2017.07.028.
Gao Q, Zhang C, Wang M, et al. Injectable pH-responsive poly (?-glutamic acid)-silica hybrid hydrogels with high mechanical strength, conductivity and cytocompatibility for biomedical applications. Polymer 2020;197:122489. https://doi.org/10.1016/j.polymer.2020.122489.
Kasbiyan H, Yousefzade O, Simiand E, et al. Antibacterial hydrogels derived from Poly(?-glutamic acid) nanofibers. Gels 2022;8:120. https://doi.org/10.3390/gels8020120.
Zhang L, Ma Y, Pan X, et al. A composite hydrogel of chitosan/heparin/poly (?-glutamic acid) loaded with superoxide dismutase for wound healing. Carbohydr Polym 2018;180:168–74. https://doi.org/10.1016/j.carbpol.2017.10.036.
Dou C, Li Z, Gong J, et al. Bio-based poly (?-glutamic acid) hydrogels reinforced with bacterial cellulose nanofibers exhibiting superior mechanical properties and cytocompatibility. Int J Biol Macromol 2021;170:354–65. https://doi.org/10.1016/j.ijbiomac. 2020.12.148.
Shi L, Yang N, Zhang H, et al. A novel poly(?-glutamic acid)/silk-sericin hydrogel for wound dressing: Synthesis, characterization and biological evaluation. Mater Sci Eng C 2015;48:533–40. https://doi.org/10.1016/j.msec.2014.12.047.
Lee YH, Lee B-W, Jung YC, et al. Application of alginate microbeads as a carrier of bone morphogenetic protein-2 for bone regeneration. J Biomed Mater Res B Appl Biomater 2019;107:286–94. https://doi.org/10.1002/jbm.b.34119.
Park H, Kang S-W, Kim B-S, et al. Shear-reversibly cross-linked alginate hydrogels for tissue engineering. Macromol Biosci 2009;9:895–901. https://doi.org/10.1002/mabi.200800376.
Mørch ÝA, Donati I, Strand BL, et al. Effect of Ca2+, Ba2+, and Sr2+ on alginate microbeads. Biomacromol 2006;7:1471–80. https://doi.org/10.1021/bm060010d.
Leong J-Y, Lam W-H, Ho K-W, et al. Advances in fabricating spherical alginate hydrogels with controlled particle designs by ionotropic gelation as encapsulation systems. Particuology 2016;24:44–60. https://doi.org/10.1016/j.partic.2015.09.004.
Zhang M, Zhao X. Alginate hydrogel dressings for advanced wound management. Int J Biol Macromol 2020;162:1414–28. https://doi.org/10.1016/j.ijbiomac.2020.07.311.
Peña C, Hernández L, Galindo E. Manipulation of the acetylation degree of Azotobacter vinelandii alginate by supplementing the culture medium with 3-(N-morpholino)-propane-sulfonic acid. Lett Appl Microbiol 2006;43:200–4. https://doi.org/10.1111/j.1472-765X.2006.01925.x.
Dudun AA, Akoulina EA, Zhuikov VA, et al. Competitive biosynthesis of bacterial alginate using Azotobacter vinelandii 12 for tissue engineering applications. Polymers 2022;14. https://doi.org/10.3390/polym14010131.
Galindo E, Peña C, Núñez C, et al. Molecular and bioengineering strategies to improve alginate and polyhydroxyalkanoate production by Azotobacter vinelandii. Microb Cell Fact 2007;6:7. https://doi.org/10.1186/1475-2859-6-7.
Aarstad OA, Stanisci A, Sætrom GI, et al. Biosynthesis and function of long guluronic acid-blocks in alginate produced by Azotobacter vinelandii. Biomacromol 2019;20:1613–22. https://doi.org/10.1021/acs.biomac.8b01796.
Sabra W, Zeng A-P, Lünsdorf H, et al. Effect of oxygen on formation and structure of Azotobacter vinelandii alginate and its role in protecting nitrogenase. Appl Environ Microbiol 2000;66:4037–44. https://doi.org/10.1128/AEM.66.9.4037-4044.2000.
Moral ÇK, Sanin FD. An investigation of agitation speed as a factor affecting the quantity and monomer distribution of alginate from Azotobacter vinelandii ATCC 9046. J Ind Microbiol Biotechnol 2012; 39: 513 – 9. https://doi.org/10.1007/s10295-011-1043-3
Moral ÇK, Do?an Ö, Sanin FD. Effect of oxygen tension and medium components on monomer distribution of alginate. Appl Biochem Biotechnol 2015;176:875–91. https://doi.org/10.1007/s12010-015-1617-z.
Díaz-Barrera A, Sanchez-Rosales F, Padilla-Córdova C, et al. Molecular weight and guluronic/mannuronic ratio of alginate produced by Azotobacter vinelandii at two bioreactor scales under diazotrophic conditions. Bioprocess Biosyst Eng 2021;44:1275–87. https://doi.org/10.1007/s00449-021-02532-8.
Moral ÇK, Y?ld?z M. Alginate production from alternative carbon sources and use of polymer based adsorbent in heavy metal removal. Int J Polymer Sci 2016;2016:1–8. https://doi.org/10.1155/2016/7109825.
Castillo T, Galindo E, Peña CF. The acetylation degree of alginates in Azotobacter vinelandii ATCC9046 is determined by dissolved oxygen and specific growth rate: studies in glucose-limited chemostat cultivations. J Ind Microbiol Biotechnol 2013;40:715–23. https://doi.org/10.1007/s10295-013-1274-6.
Díaz-Barrera A, Maturana N, Pacheco-Leyva I, et al. Different responses in the expression of alginases, alginate polymerase and acetylation genes during alginate production by Azotobacter vinelandii under oxygen-controlled conditions. J Ind Microbiol Biotechnol 2017;44:1041–51. https://doi.org/10.1007/s10295-017-1929-9.
Castillo T, Heinzle E, Peifer S, et al. Oxygen supply strongly influences metabolic fluxes, the production of poly(3-hydroxybutyrate) and alginate, and the degree of acetylation of alginate in Azotobacter vinelandii. Process Biochem 2013;48:995–1003. https://doi.org/10.1016/j.procbio.2013.04.014.
Peña C, Trujillo-Roldán MA, Galindo E. Influence of dissolved oxygen tension and agitation speed on alginate production and its molecular weight in cultures of Azotobacter vinelandii. Enzyme Microb Technol 2000;27:390–8. https://doi.org/10.1016/S0141-0229(00)00221-0.
Lozano E, Galindo E, Peña CF. Oxygen transfer rate during the production of alginate by Azotobacter vinelandii under oxygen-limited and non oxygen-limited conditions. Microb Cell Fact 2011;10:13. https://doi.org/10.1186/1475-2859-10-13.
Flores C, Moreno S, Espín G, et al. Expression of alginases and alginate polymerase genes in response to oxygen, and their relationship with the alginate molecular weight in Azotobacter vinelandii. Enzyme Microb Technol 2013;53:85–91. https://doi.org/10.1016/j.enzmictec.2013.04.010.
Gómez-Pazarín K, Flores C, Castillo T, et al. Molecular weight and viscosifyingpower of alginates produced in Azotobacter vinelandii cultures in shake flasks under low power input. J Chem Technol Biotechnol 2016;91:1485–92. https://doi.org/10.1002/jctb.4747.
Díaz-Barrera A, Peña C, Galindo E. The oxygen transfer rate influences the molecular mass of the alginate produced by Azotobacter vinelandii. Appl Microbiol Biotechnol 2007;76:903–10. https://doi.org/10.1007/s00253-007-1060-3.
Díaz-Barrera A, Silva P, Berrios J, et al. Manipulating the molecular weight of alginate produced by Azotobacter vinelandii in continuous cultures. Bioresour Technol 2010;101:9405–8. https://doi.org/10.1016/j.biortech.2010.07.038.
Díaz-Barrera A, Aguirre A, Berrios J, et al. Continuous cultures for alginate production by Azotobacter vinelandii growing at different oxygen uptake rates. Process Biochem 2011;46:1879–83. https://doi.org/10.1016/j.procbio.2011.06.022.
Díaz-Barrera A, Soto E, Altamirano C. Alginate production and alg8 gene expression by Azotobacter vinelandii in continuous cultures. J Ind Microbiol Biotechnol 2012;39:613–21. https://doi.org/10.1007/s10295-011-1055-z.
Díaz-Barrera A, Gutierrez J, Martínez F, et al. Production of alginate by Azotobacter vinelandii grown at two bioreactor scales under oxygen-limited conditions. Bioprocess Biosyst Eng 2014;37:1133–40. https://doi.org/10.1007/s00449-013-1084-2.
Flores C, Díaz-Barrera A, Martínez F, et al. Role of oxygen in the polymerization and depolymerization of alginate produced by Azotobacter vinelandii. J Chem Technol Biotechnol 2015;90:356–65. https://doi.org/10.1002/jctb.4548.
Pacheco-Leyva I, Pezoa FG, Díaz-Barrera A. Alginate biosynthesis in Azotobacter vinelandii: Overview of molecular mechanisms in connection with the oxygen availability. Int J Polym Sci 2016;2016:2062360. https://doi.org/10.1155/2016/2062360.
Yao J, Jing J, Xu H, et al. Investigation on enzymatic degradation of ?-polyglutamic acid from Bacillus subtilis NX-2. J Mol Catal B Enzym 2009;56:158–64. https://doi.org/10.1016/j.molcatb.2007.12.027.
Luo Z, Guo Y, Liu J, et al. Microbial synthesis of poly-?-glutamic acid: current progress, challenges, and future perspectives. Biotechnol Biofuels 2016;9:134. https://doi.org/10.1186/s13068-016-0537-7.
Anju AJ, Sindhu R, Parameswaran B, et al. Production, characterization, and application of microbial poly-g-glutamic acid. In: Varjani SJ, Parameswaran B, Kumar S, Khare SK, editors. Biosynthetic Technology and Environmental Challenge. Singapore: Springer; 2018, p. 105 – 126.
Candela T, Fouet A. Poly-gamma-glutamate in bacteria. Mol Microbiol 2006;60:1091–8. https://doi.org/10.1111/j.1365-2958.2006.05179.x.
Candela T, Moya M, Haustant M, et al. Fusobacterium nucleatum, the first Gram-negative bacterium demonstrated to produce polyglutamate. Can J Microbiol 2009;55:627–32. https://doi.org/10.1139/W09-003.
Kobayashi K. Plant methyl salicylate induces defense responses in the rhizobacterium Bacillus subtilis. Environ Microbiol 2015;17:1365–76. https://doi.org/10.1111/1462-2920.12613.
Schallmey M, Singh A, Ward OP. Developments in the use of Bacillus species for industrial production. Can J Microbiol 2004;50:1–17. https://doi.org/10.1139/w03-076.
Shih I-L, Van Y-T. The production of poly-(?-glutamic acid) from microorganisms and its various applications. Bioresour Technol 2001;79:207–25. https://doi.org/10.1016/S0960-8524(01)00074-8.
Wu Q, Xu H, Xu L, et al. Biosynthesis of poly(?-glutamic acid) in Bacillus subtilis NX-2: Regulation of stereochemical composition of poly(?-glutamic acid). Process Biochem 2006;41:1650–5. https://doi.org/10.1016/j.procbio.2006.03.034.
Ashiuchi M, Misono H. Biochemistry and molecular genetics of poly-?-glutamate synthesis. Appl Microbiol Biotechnol 2002;59:9–14. https://doi.org/10.1007/s00253-002-0984-x.
Ashiuchi M, Shimanouchi K, Nakamura H, et al. Enzymatic synthesis of high-molecular-mass poly-?-glutamate and regulation of its stereochemistry. Appl Environ Microbiol 2004;70:4249–55. https://doi.org/10.1128/AEM.70.7.4249-4255.2004.
Ashiuchi M, Kamei T, Misono H. Poly-?-glutamate synthetase of Bacillus subtilis. J Mol Catal B Enzym 2003;23:101–6. https://doi.org/10.1016/S1381-1177(03)00076-6.
Cromwick A-M, Birrer GA, Gross RA. Effects of pH and aeration on ?-poly(glutamic acid) formation by Bacillus licheniformis in controlled batch fermentor cultures. Biotechnol Bioeng 1996;50:222–7. https://doi.org/10.1002/(SICI)1097-0290(19960420)50:2<222::AID-BIT10>3.0.CO;2-P.
Flores C, Medina?Valdez A, Peña C, et al. Oxygen transfer rate determines molecular weight and production of poly(?-glutamic acid) as well as carbon utilization by Bacillus velezensis 83. J Chem Technol Biotechnol 2020;95:2383–92. https://doi.org/10.1002/jctb.6420.
Obst M, Steinbüchel A. Microbial Degradation of Poly(amino acids). Biomacromol 2004;5:1166–76. https://doi.org/10.1021/bm049949u .
Zhang D, Feng X, Li S, et al. Effects of oxygen vectors on the synthesis and molecular weight of poly(?-glutamic acid) and the metabolic characterization of Bacillus subtilis NX-2. Process Biochem 2012;47:2103–9. https://doi.org/10.1016/j.procbio.2012.07.029.
Sha Y, Qiu Y, Zhu Y, et al. CRISPRi-based dynamic regulation of hydrolase for the synthesis of poly-?-glutamic acid with variable molecular weights. ACS Synth Biol 2020;9:2450–9. https://doi.org/10.1021/acssynbio.0c00207.
Feng J, Shi Q, Zhou G, et al. Improved production of poly-?-glutamic acid with low molecular weight under high ferric ion concentration stress in Bacillus licheniformis ATCC 9945a. Process Biochem 2017;56:30–6. https://doi.org/10.1016/j.procbio.2017.02.017.
Cromwick A-M, Gross RA. Effects of manganese (II) on Bacillus licheniformis ATCC 9945A physiology and ?-poly(glutamic acid) formation. Int J Biol Macromol 1995;17:259–67. https://doi.org/10.1016/0141-8130(95)98153-P.
Bajaj IB, Singhal RS. Effect of aeration and agitation on synthesis of poly (?-glutamic acid) in batch cultures of Bacillus licheniformis NCIM 2324. Biotechnol Bioprocess Eng 2010;15:635–40. https://doi.org/10.1007/s12257-009-0059-2.
Zhao C, Zhang Y, Wei X, et al. Production of ultra-high molecular weight poly-?-glutamic acid with Bacillus licheniformis P-104 and characterization of its flocculation properties. Appl Biochem Biotechnol 2013;170:562–72. https://doi.org/10.1007/s12010-013-0214-2.
Halmschlag B, Steurer X, Putri SP, et al. Tailor-made poly-?-glutamic acid production. Metab Eng 2019;55:239–48. https://doi.org/10.1016/j.ymben.2019.07.009.
Shimizu K, Nakamura H, Ashiuchi M. Salt-inducible bionylon polymer from Bacillus megaterium. Appl Environ Microbiol 2007;73:2378–9. https://doi.org/10.1128/AEM.02686-06.
Kubota H, Matsunobu T, Uotani K, et al. Production of poly(gamma-glutamic acid) by Bacillus subtilis F-2-01. Biosci Biotechnol Biochem 1993:1212–1213. doi: 10.1271/bbb.57.1212.
Ogasawara Y, Shigematsu M, Sato S, et al. Involvement of peptide epimerization in poly-?-glutamic acid biosynthesis. Org Lett 2019; 21:11: 3972-75. doi: 10.1021/acs.orglett.9b01121
Sawada K, Araki H, Takimura Y, et al. Poly-L-gamma-glutamic acid production by recombinant Bacillus subtilis without pgsA gene. AMB Express. 2018; 8(1):110. doi: 10.1186/s13568-018-0636-x.

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright (c) 2023 Electronic Journal of Biotechnology